Our Platform
Seres’ pioneering science, paired with powerful drug development and manufacturing capabilities, underpins its unique therapeutics platform.
Transforming rigorous scientific insights into groundbreaking new medicines
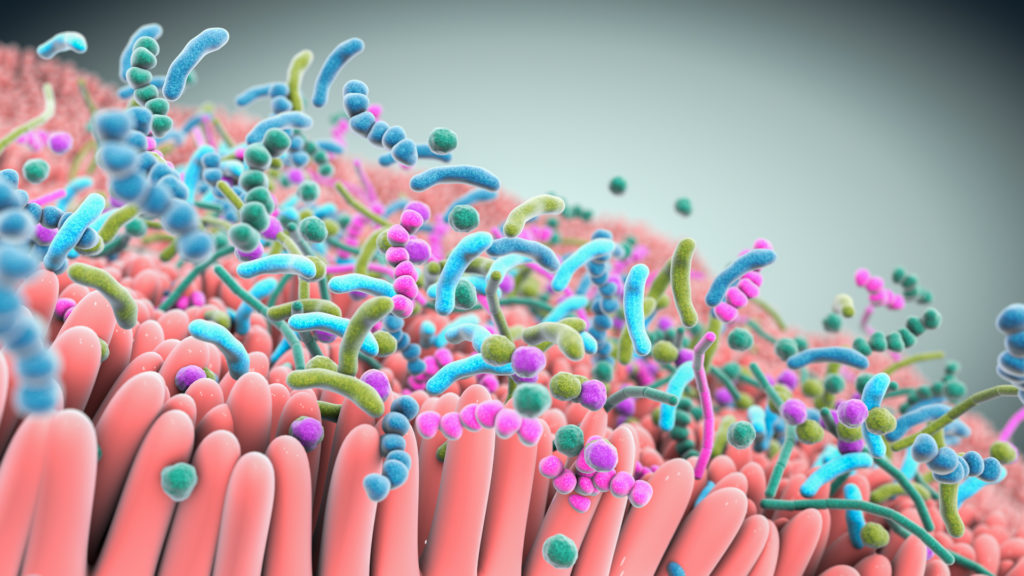
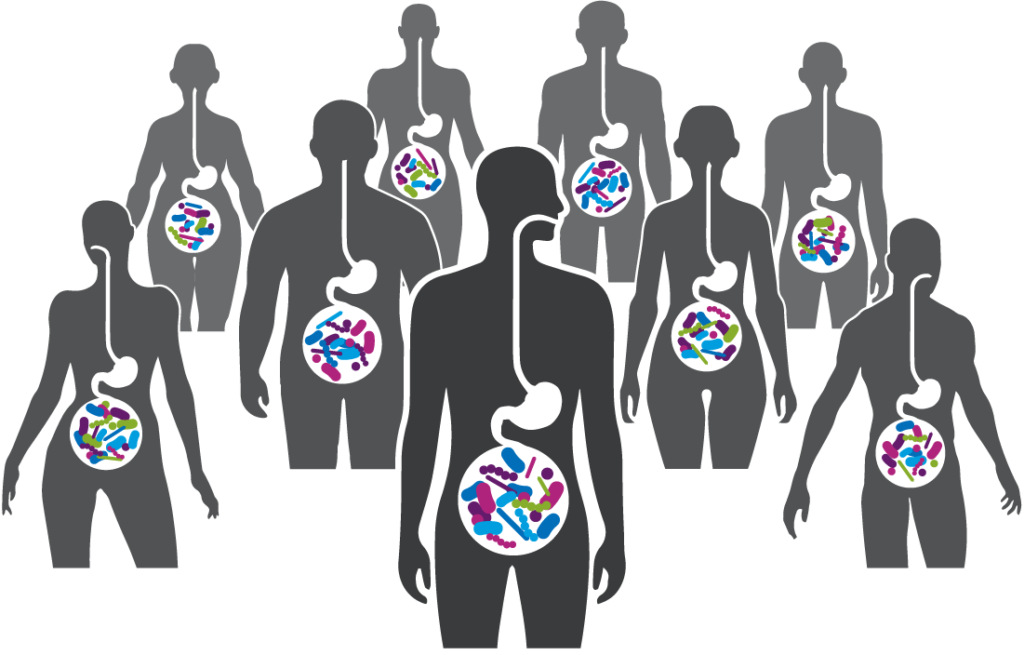
A robust body of research, which includes work by Seres and our collaborators, has revealed the integral role the gut microbiome plays in overall human health. Increasingly, the gut microbiome is understood as a pillar of essential functions such as protection against potential invaders, immune and inflammatory responses, metabolism, and neurological signaling.
Bacteria, a major component of the gut microbiome, can affect these various functions by producing metabolites and peptides that interact with other microbes and the host. Scientific understanding of the role of the microbiome and microbe-associated metabolites has advanced tremendously and is enabling the development of therapeutics that may treat a variety of serious diseases.
Over the past decade, Seres has pioneered the translation of microbiome insights into an entirely new class of potential new medicines. This includes the development and commercialization of the first-ever FDA-approved oral live biotherapeutic, VOWST™*.
Our live biotherapeutics are comprised of rationally selected consortia of bacteria in oral capsules that are designed and optimized to have specific functional pharmacological properties. This includes preventing infections, restoring epithelial barrier integrity, and modulating immune functions. We believe that these therapies could provide benefit to medically vulnerable populations that are at risk for bacterial infections and other potentially life-threatening complications.
*VOWST™ was sold to Nestlé Health Science in September 2024.
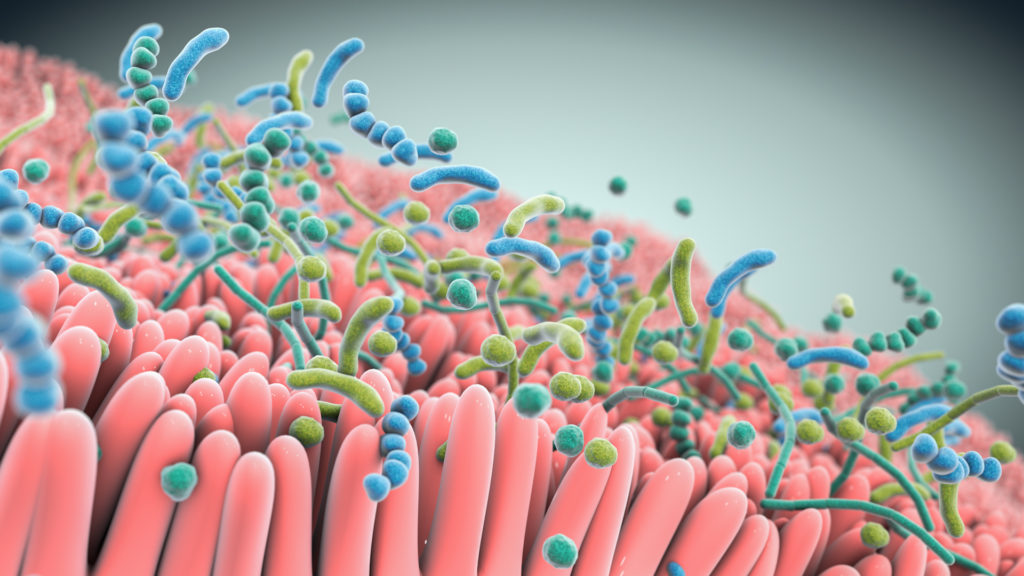
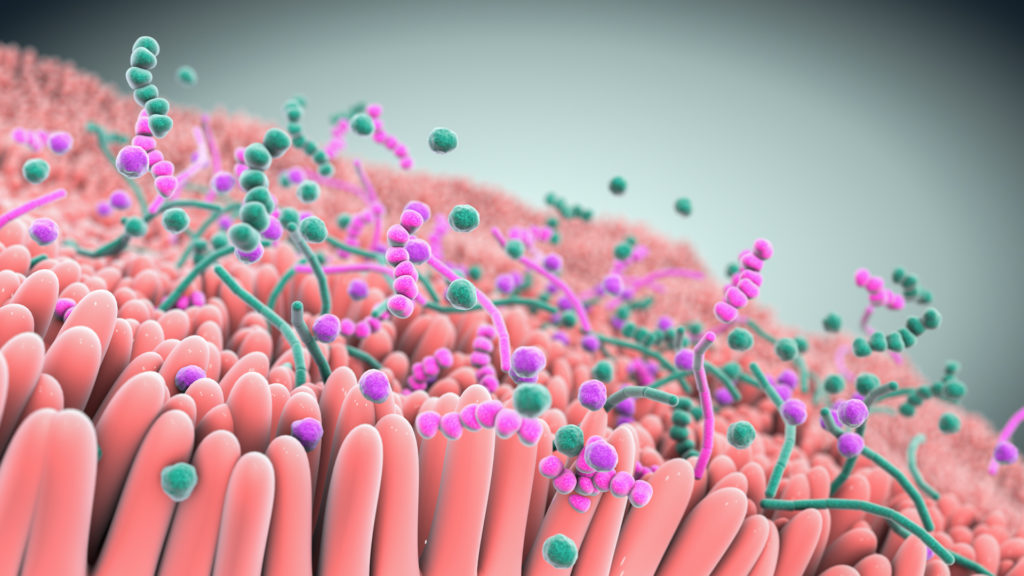
Studies show that live biotherapeutics can drive pharmacological effects across multiple pathways simultaneously.
Our approach:
Delivering a multifunctional
consortium of bacteria
At the heart of our approach are multifunctional consortia of bacteria that are designed to functionally interact with host cells and tissues to treat disease.
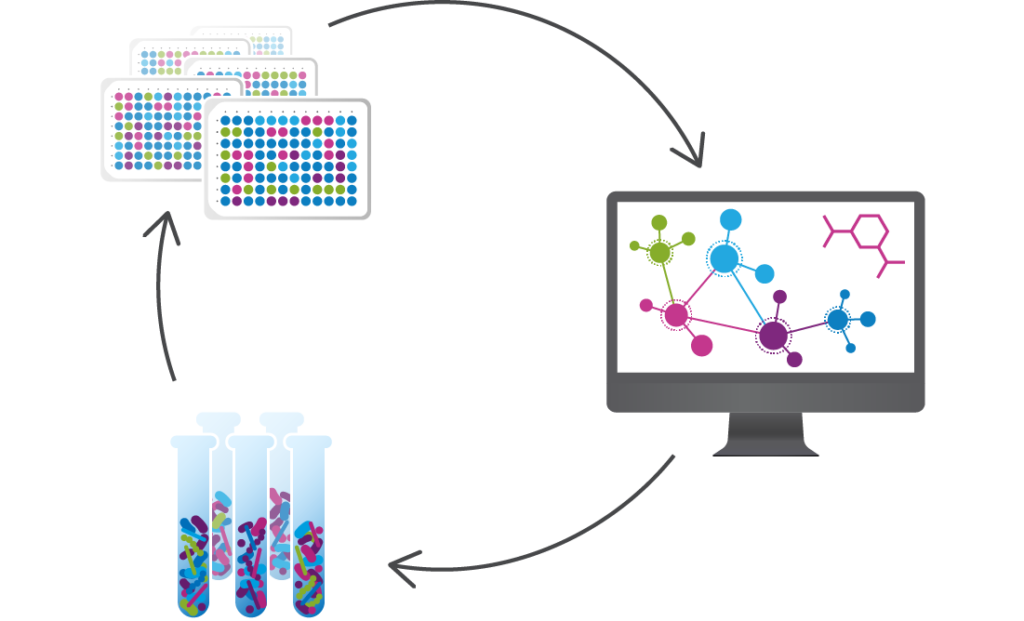
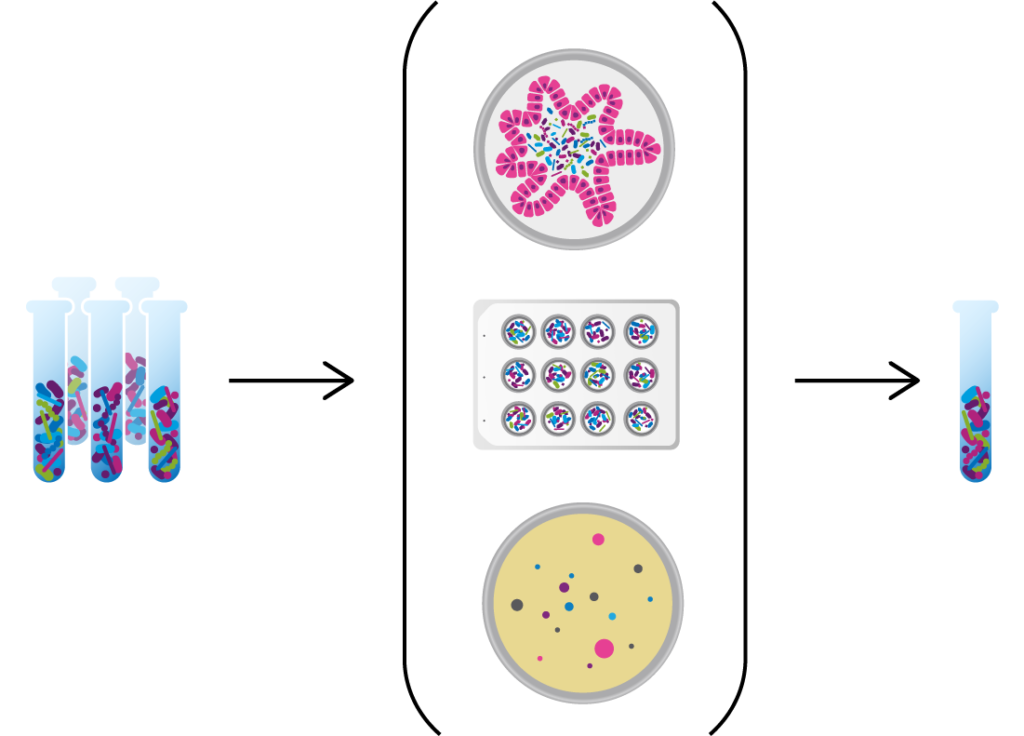
We use data from clinical studies to discover the microbes and microbe-associated metabolites that functionally interact with human cells and tissues to see if they can be targeted to treat infections and other diseases. We then utilize advanced computational tools and proprietary functional assays to design consortia of commensal bacteria that modulate these targets and act on multiple microbial and host functions.
Seres is harnessing these defined consortia, or collections, of bacteria to modulate function pathways in the gastrointestinal tract and systemically modulate microbiome function. Instead of targeting just one disease pathway, a benefit of using bacterial consortia is that they are multifunctional. This means they might have multiple pharmacological effects and modulate numerous functional pathways within the body to achieve therapeutic impact.
Our research strategy:
The Live Biotherapeutics
Engine
Seres has built an unprecedented Biotherapeutics Engine to design, test, and manufacture multifunctional bacterial consortia.